What’s in my atoms?
This What’s in my tray? activity was created and supplied by Maureen Wade, The Victory Academy, Kent.
Explore atomic structure, the relationships between sub-atomic particles and learn how to determine atomic mass and atomic number.
Learning outcomes
You will be able to:
- Describe the structure of atoms.
- Describe the masses and charges of sub-atomic particles.
- Relate the number of subatomic particles to atomic number and atomic mass.
- Describe electronic structure.
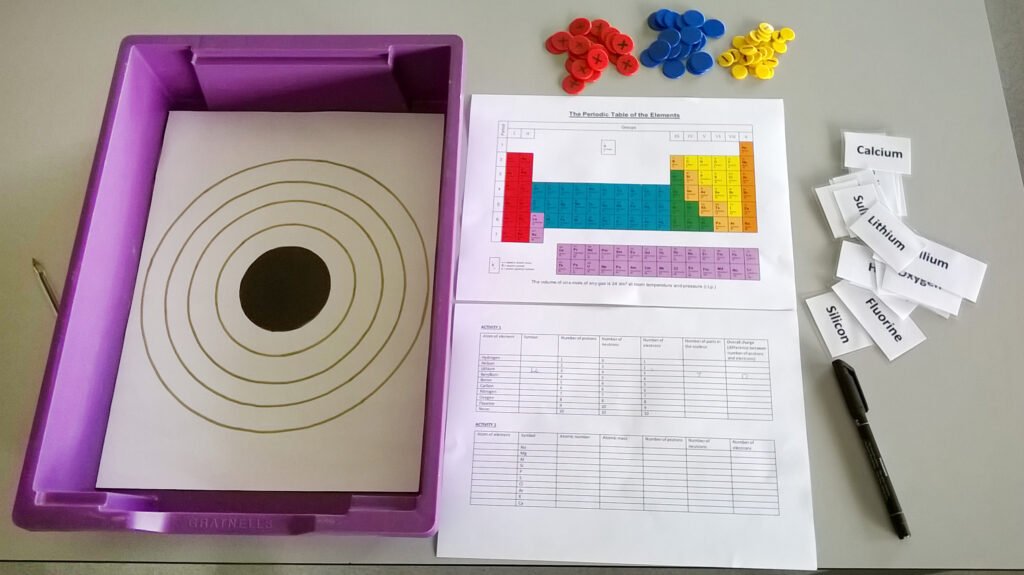
You will need (per person or pair of participants):
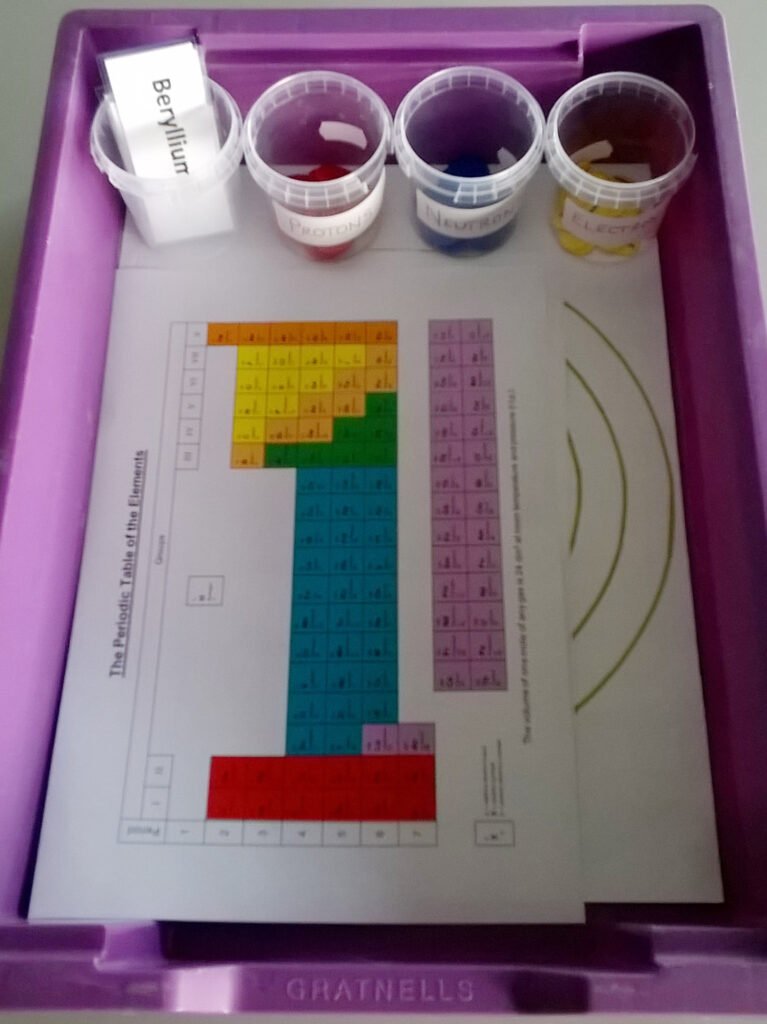
- 1 x Gratnells shallow tray
- 4 x Small SortED insert modules
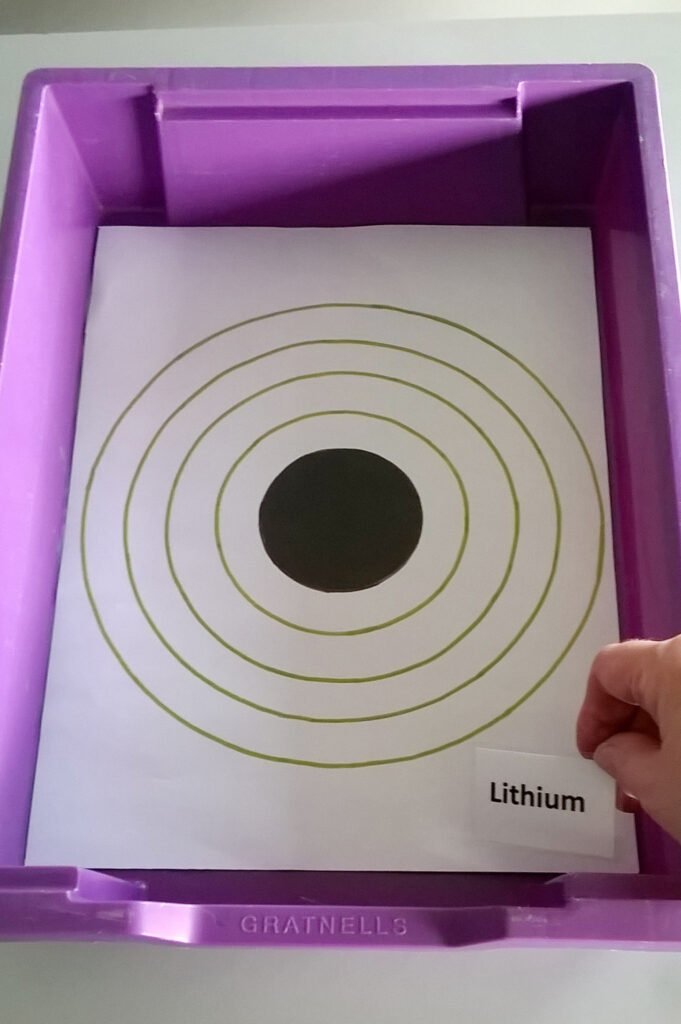
- 1 x Atomic structure diagram inlay (Atomic Structure Diagram tray inlay.pdf)
- 20 x Large counters in each of blue and red
- 20 x Small counters in yellow
- 1 x Set of element name labels (Element name labels.docx)
- 1 x Activity guidance sheet (What’s In My Atoms Activity Guidance Sheet.docx)
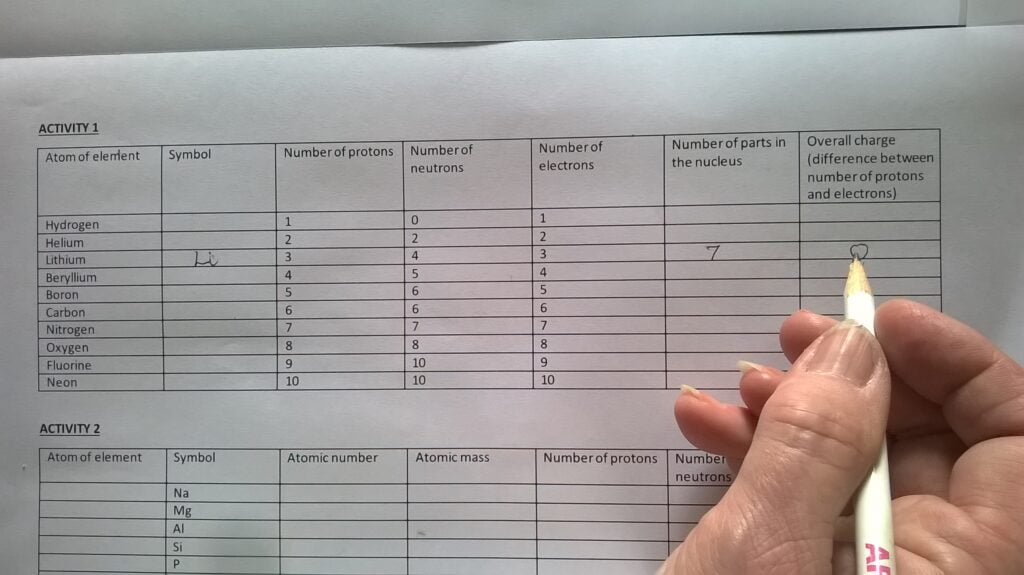
- 1 x Activity tables (Activity Tables.docx)
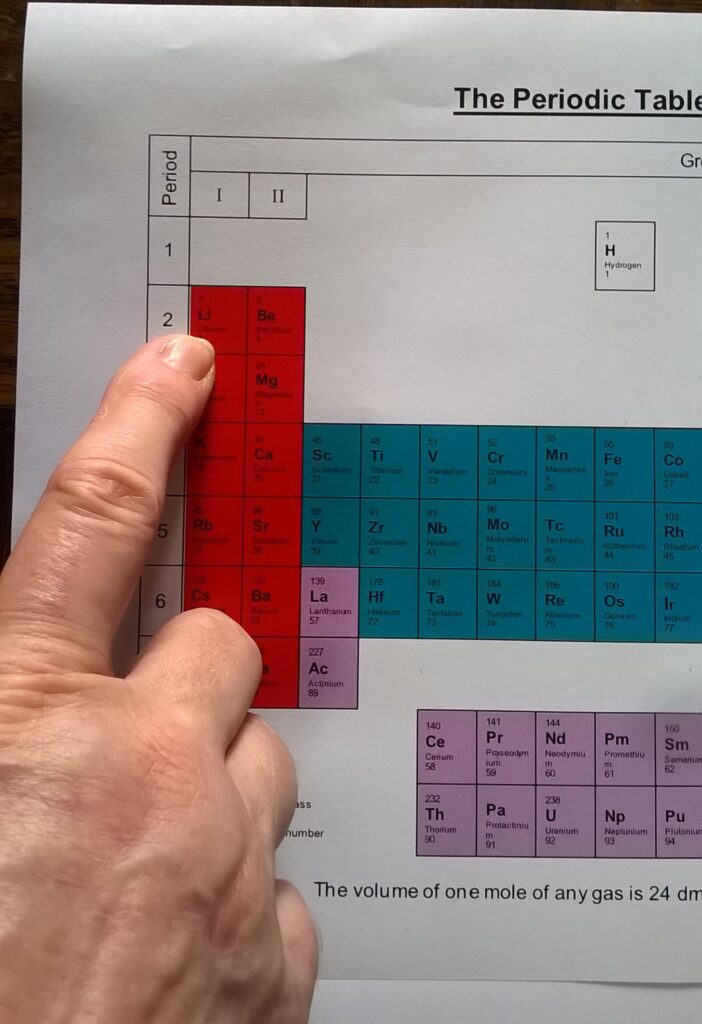
- 1 x Periodic table (The Periodic Table (coloured).doc)
For preparation use only:
- 1 x Marker pen for writing on the counters
Preparation:
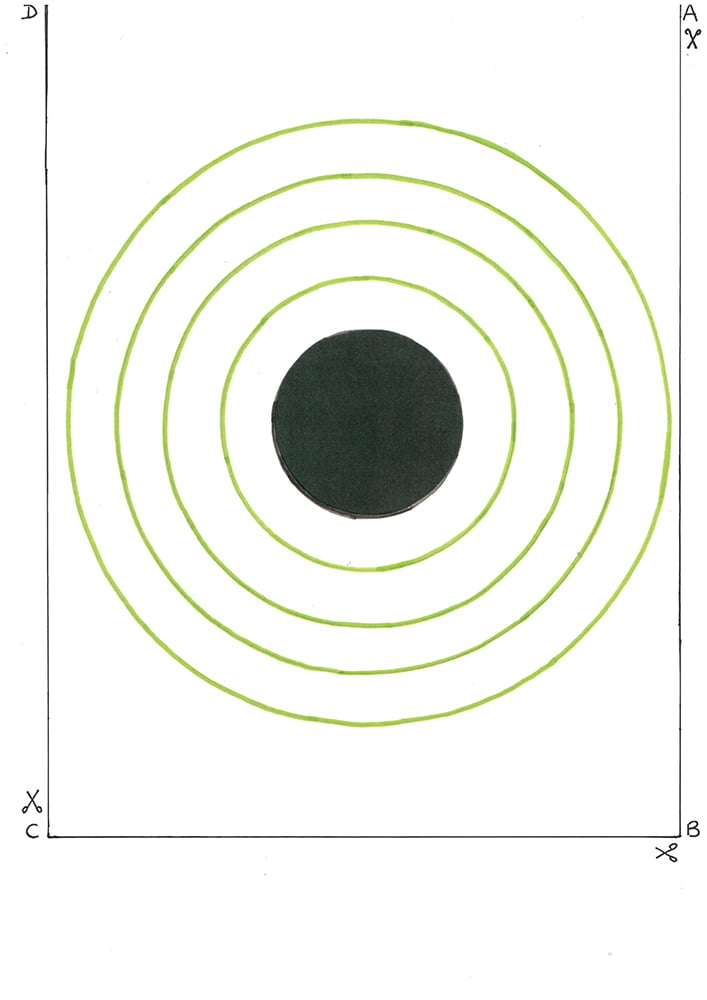
- Print the Atomic structure diagram inlay (print as A3 and trim to tray size by cutting along the solid black line ABCD).
- Print the activity tables and the periodic table.
- Print the element name labels, cut along the solid black lines to separate, and then laminate.
- Sort counters and element name labels into the SortED insert modules. Use larger counters for protons and neutrons and smaller counters for electrons to help with students’ perception that electrons are smaller than the other subatomic particles.
- Mark the counters with the associated charge using the marker pen.
What to do:
Introductory Class Discussion
Determine prior knowledge and impart new knowledge through a question and answer session. Ask the following questions:
- What is an atom?
- What are the three main parts of an atom?
- Where are these parts of the atom found?
- What are electron shells and how many electrons can they hold?
- What charges are associated with these parts?
Or use the discussion questions as a round-up at the end of the practical activity.
Activity 1
- Using activity table 1, the Atomic structure diagram inlay, and the counters, model the atoms listed in activity table 1 and complete the empty columns in this table.
- Label the model with the corresponding element name label.
- Consult the periodic table, identify the symbols for the elements and notice any patterns between the periodic table and their atomic model.
What is happening:
The students should recognise that atomic number is the same as the number of protons and that atomic mass is the same as the number of protons plus neutrons.
Students should also notice that atoms have no overall charge and the mass of the electron is so small that it does not contribute to atomic mass.
Activity 2
Referring to the periodic table for symbols, atomic number, and atomic mass complete the following tasks:
- Working in the tray and using the atomic structure diagram inlay, model the atoms listed in activity table 2,
- Complete the empty columns in activity table 2.
- Label the model with the corresponding element name label.
Plenary
Fill in the blank spaces with words from the box

The smallest unit of matter forming a chemical element is an ……………. There are ………………..parts that make up an atom, these are …………….. which have a positive charge, electrons which have a …………………charge and ……………….. which have …………….. charge. The nucleus of the atom contains ………………. and ………………. Orbiting the nucleus are ……………… which can be found at different energy levels. The number of protons in an atom is equal to the atomic ……………… and the number of protons plus the number of ……………… is equal to the atomic ……………… The mass of ………………….is negligible. The maximum number of electrons in the ……………….. shell is two. The second and third shells may contain up to ………………electrons.
Other things to try…
- The activity can be modified for differentiated teaching by increasing the number of given facts in each activity table, by reducing the number of elements being considered or by working in groups.
- Use the plenary to summarise and assess learning objectives.
- For more able students, discuss why the periodic table has an atomic mass of 35.5 for chlorine and not a whole number.
Curriculum links:
- AQA GCSE Chemistry 4.1.1.4 Relative Electrical Charges of Sub-atomic Particles
- AQA GCSE Chemistry 4.1.1.5 Size and mass of Atoms
- AQA GCSE Chemistry 4.1.1.6 Relative Atomic Mass
- AQA GCSE Chemistry 4.1.1.7 Electronic Structure
Health & Safety
As with all Gratnells Learning Rooms What’s in my tray? activities, you should carry out your own risk assessment prior to undertaking any of the activities or demonstrations.